Mycotoxins and endotoxins are naturally occurring toxins posing a serious threat to animal production. Many harmful effects—leaky gut, mouth lesions, damaged villi—are linked to toxins’ capacity to induce oxidative stress in animals, negatively impacting performance and health.
Mycotoxins and endotoxins share similarities in their ability to induce oxidative stress. Both can generate reactive oxygen species (ROS), disrupt antioxidant defense systems, and trigger inflammatory responses that contribute to oxidative damage. However, mycotoxins (which are produced by fungi) can directly generate ROS and initiate lipid peroxidation, while endotoxins (produced by gram-negative bacteria) primarily induce oxidative stress through inflammatory responses and ROS production associated with immune activation.
Mycotoxins are toxic compounds naturally produced by certain types of molds (fungi). Mold growth typically occurs in warm and humid conditions and can develop before or after harvest, during storage, or even in the finished feed. Molds grow on numerous foodstuffs—such as cereals, dried fruits, nuts, and spices—and when stressed, can produce secondary metabolites called mycotoxins. Most mycotoxins are chemically stable and can survive food processing. Some generally known symptoms of myotoxicity in livestock include vomiting, decreased feed intake, reduced growth, depressed immunity, fertility problems, and increased mortality.
Endotoxins or lipopolysaccharides (LPS) are structural components of gram-negative bacteria. They are part of the outer membrane and are mainly released when bacteria are lysed—the cell wall is broken down—due to antibiotics or the immune response. LPS consists of three elements (Figure 1): Lipid A (a hydrophobic component that serves as an anchor when a bacterium is invading a host cell), a core (an oligosaccharide), and O-antigen (a hydrophilic component projecting into the extracellular space of the intact bacteria cells). Studies have found Lipid A is responsible for most of the toxicity caused by endotoxins. Common signs of endotoxins include an inflammatory response leading to fever and diarrhea.
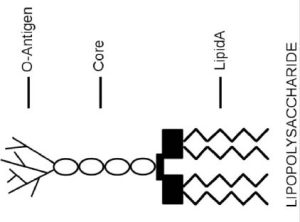
Figure 1. An LPS Structure
After the ingestion of feed contaminated with mycotoxins, the gastrointestinal tract is the first organ targeted. Effects on intestinal epithelial cells include changed mucus production, altered cytokine fabrication, decreased cell proliferation, and compromised intestinal barrier function. After crossing the epithelial cells, mycotoxins are taken up in the blood and transported to the liver. In the liver, mycotoxins are metabolized into secondary metabolites. Frequently, this will reduce the toxic effects of the original mycotoxin, however, sometimes the result is the opposite. For example, Zearalenone (ZEA)—a mycotoxin with estrogenic activity—can be converted into two metabolites, α- zearalenol (500 times more estrogenic than ZEA) or β- zearalenol (16 times less estrogenic than ZEA). Another example is Aflatoxin B1 being converted into Aflatoxin M1, which is a risk to consumers who ingest milk with high levels of Aflatoxin M1. After passing the liver, mycotoxins are systemically distributed throughout the body, impacting the immune system and all organs.
Endotoxins are normally not present in feed but are produced in the gastrointestinal tract when there is an overgrowth of gram-negative bacteria. The physiological activities of LPS are mediated mainly by the Lipid A component of LPS. Lipid A is a powerful biological response modifier that can stimulate the mammalian immune system. LPS stimulates localized or systemic inflammation via the activation of receptors. Additionally, LPS—and associated inflammation—can regulate intestinal epithelial function by altering epithelial barrier integrity as well as nutrient transport and utilization.
The detrimental action of both endotoxins and mycotoxins has some common elements. One is the induction of oxidative stress. Under normal conditions, free radicals or ‘Reactive Oxygen Species’ (ROS) are produced as part of the standard metabolic process. These highly unstable and chemically reactive molecules are rapidly eliminated by the natural antioxidant system to avoid potential damage. However, when exposed to mycotoxins and/or endotoxins, cellular ROS concentrations exceed the level of naturally occurring antioxidants, which results in oxidative stress. Excess ROS will induce a damaging chain reaction, causing destruction to nucleic acids, proteins, and lipids. As these components are the basic molecules for all metabolic processes, ROS directly affects the viability of cells, animal health, and performance.
Oxidative stress in animals can be determined using different parameters such as the half haemolysis time of blood cells (HT50), glutathione (GSH), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), or superoxide dismutase activity (SOD). Table 1 shows the impact of oxidative stress levels caused by mycotoxins in piglets. As indicated by different blood parameters, piglets exposed to the maximum allowed EU level of feed contamination of DON (0.9 ppm) experienced more oxidative stress. These higher oxidative stress levels resulted in lower feed intake and reduced growth performance—negatively affecting profitability.
| Negative control | 0.9 ppm DOM | |
|---|---|---|
| HT50 (min)* | 90.92 | 87.51 (-3.8%) |
| GSH-Px, U/mg protein** | 6.26 | 6.62 (+5.8%) |
| MDA, nmol/g protein*** | 41.75 | 44.86 (+7.4%) |
A similar effect on oxidative stress is demonstrated in Table 2. An LPS injection was given and resulted in reduced activities of SOD and GSH- Px in the jejunum of the pigs. A significantly greater (P< 0.05) content of MDA in the jejunal mucosa was observed in the LPS group compared with the control group.
| Control | LPS | |
|---|---|---|
| SOD, U/mg protein* | 95.85 + 3.13a | 63.12 + 2.97b |
| GSH-Px, U/mg protein** | 72.11 + 3.51a | 53.99 + 3.26b |
| MDA, nmol/g protein*** | 0.95 + 0.16a | 1.51 + 0.25b |
After mycotoxins and endotoxins come into contact with epithelial cells or are absorbed, the gastrointestinal tract is heavily impacted by the induction of oxidative stress. Besides the negative effects on all cellular processes, oxidative stress has an enormous impact on intestinal barrier function (Figure 2). The intestinal barrier is formed by a layer of epithelial cells covered with mucus. Tight junction proteins form a physical barrier between two adjacent epithelial cells to prevent paracellular absorption of undesired substances such as toxins or pathogens. When exposed to free radicals, oxidative stress harms this intestinal barrier function by modifying the cellular proteins; this is commonly referred to as ‘leaky gut’. In ex vivo and in vitro studies, measuring trans-epithelial electrical resistance (TEER), DON, and fumonisin B1 was shown to increase the permeability of the intestinal epithelial layer of pigs and poultry. The compromised intestinal barrier function results in increased permeability for toxins, pathogens, and feed-associated antigens. As per endotoxins, Aschenbach et al. (2003), used intestinal epithelial cell lines and concluded that an abnormal increase in luminal endotoxin induces cell apoptosis, which subsequently disrupts tight junction protein zonula occludens-1 as well as increases the production of nitric oxide, leading to increased mucosal permeability.
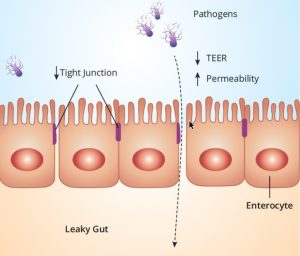
Figure 2: Oxidative stress results in impaired intestinal barrier function
The control of mycotoxins starts in the field and in storage with quality control of raw materials. However, as many research studies have shown, it seems inevitable that mycotoxins will contaminate feeds. On top of this, a quality management system does not avoid the risk of endotoxins, which are naturally produced within the animal’s gastrointestinal tract. Therefore, a holistic strategy of binding toxins to reduce oxidative stress and protect the intestinal barrier function is needed to support the animal coping with toxins. This approach enables feed producers and farmers to avoid excessive oxidative stress caused by myco- and endotoxins, resulting in optimal performance and peace of mind.
Would you like to be kept informed of our latest developments? Register here and stay up to date.
"*" indicates required fields
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
