Ergot bodies, which are produced by fungi in the Claviceps genera, particularly C. purpurea, are commonly referred to as ergot. These bodies store toxic alkaloids produced by the fungi. The severity and occurrence of ergot infection in grains vary from year to year. It is most prevalent in years with increased moisture available at the soil surface in spring and early summer or with wet weather during the flowering stage of cereal crops (Pearse, 1999).
Several cereal grain species, including rye, wheat, triticale, barley, oats, and others, are susceptible to ergot infection, resulting in the production of ergot bodies. Seed screening and cleaning processes can remove these bodies. However, if not removed, the presence of ergot bodies in grain screenings still poses a health risk for livestock. To mitigate this risk, there are currently regulations that specify the permissible quantities of ergot bodies in grain and feed (Table 1).
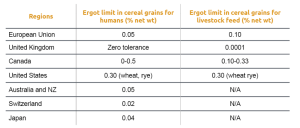
Table 1: Ergot concentrations allowed in grain and feed (Scott, 2009)
The concentration of ergot alkaloids in the grain depends on the content of sclerotia. The European Food Safety Authority (EFSA) has reported a strong correlation between the content of sclerotia and the levels of ergot alkaloids, with coefficients ranging from 0.806 for rye grains to 0.972 for triticale grains. However, the absence of sclerotia does not necessarily exclude the presence of ergot alkaloids. Some samples without identified sclerotia still showed measurable levels of ergot alkaloids.
The effects of ergot toxicity vary among different livestock species. Poultry seems to tolerate higher dietary alkaloid concentrations compared to ruminants or swine (Thompson, 2016). Common clinical symptoms of ergot poisoning include vasoconstriction, which may progress to gangrene, disruption of reproduction, abortion, neurotoxic signs such as feed refusal, dizziness, convulsions, agalactia, and adverse effects on the cardiovascular system. Typically, these symptoms are associated with high contamination levels exceeding regulatory or recommended limits.
Reports have shown that ergot concentrations close to regulations can have detrimental effects. In a swine trial where animals were exposed to doses with concentrations near EU regulations (1.2 and 2.5 gr sclerotia/kg feed) for 28 days, significant results were observed. Animals fed with the higher dosage showed decreased feed intake in the second week, while those fed with the lower dosage reported a reduction in the last 14 days of the experiment. Although no clinical signs of acute toxicity were reported, various organs were affected. The liver exhibited tissue alterations, including the development of inflammatory infiltrates, while the jejunum showed reduced villi height and increased damage to the epithelium (Maruo et al., 2018).
Other studies (Oresanya et al., 2003) reported a dose-response reduction in average daily feed intake in the last week of a 28 days trial with weaned pigs fed feed containing ergot concentrations ranging from 0.05 to 1.00% of the diets. The reduction in feed intake has been linked to the aromatic properties of ergot alkaloids (Whittemore et al., 1977). Because of several reports of detrimental effects with ergot contamination close to regulation limits, different authors have suggested a more practical table of recommendations taking into consideration the actual alkaloid content rather than ergot bodies.
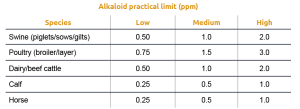
Table 2: Recommended practical ergot alkaloid levels for various livestock species (Coufal-Majewski, 2016)

At the feed mill level, the recommended approach for preventing ergot contamination is similar to addressing any mycotoxin contamination. It involves implementing effective quality control measures for incoming raw materials, with a strong emphasis on proper sampling protocols. In the subsequent feed manufacturing process, various techniques can help reduce the risk, such as grain cleaning methods (e.g., scalpers, shaker decks) that remove impurities, as well as specific grain cleaning equipment like gravitational separators and color sorters. These methods aid in minimizing the presence of ergot bodies. On the farm level, it is advised to remove any feed that has been affected by ergot.
Similarly to addressing other mycotoxin contaminations, the use of mycotoxin binders is an alternative approach. Although there are limited reports of in vivo studies testing mycotoxin binders specifically for ergot, evaluating the in vitro binding efficacy–as done with other mycotoxins–can provide insights into a product’s potential to reduce the effects of ergot contamination when administered to farm animals. Therefore, conducting in vitro binding tests is highly valuable for distinguishing between different commercially available toxin binders and selecting a binder that can effectively tackle ergot contaminations in animal feed, thus bringing added value to farming operations.

Would you like to be kept informed of our latest developments? Register here and stay up to date.
"*" indicates required fields
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
