Endotoxins are common in the gastrointestinal tract (GIT) of animals, particularly the large intestine with enterobacteria as the most important endotoxin-containing bacteria. In addition to causing damage to the GIT, when endotoxins are excreted through the feces, they attach to dust particles and become a bioaerosol found in feed, litter, and feces that cause inflammatory reactions in the respiratory tract of the animals that inhaled them.
Endotoxins are—together with phospholipids and membrane-bound proteins—structural components of the outer membrane of gram-negative bacteria. Endotoxins, also called lipopolysaccharides (LPS), are only found in gram-negative bacteria, like E. coli. What makes gram-negative bacteria differ from gram-positive bacteria is the structure of the cell envelope. Gram-positive bacteria have a cytoplasmic and a peptidoglycan outer membrane. In addition to these layers, gram-negative bacteria have an extra outer membrane (Figure 1).
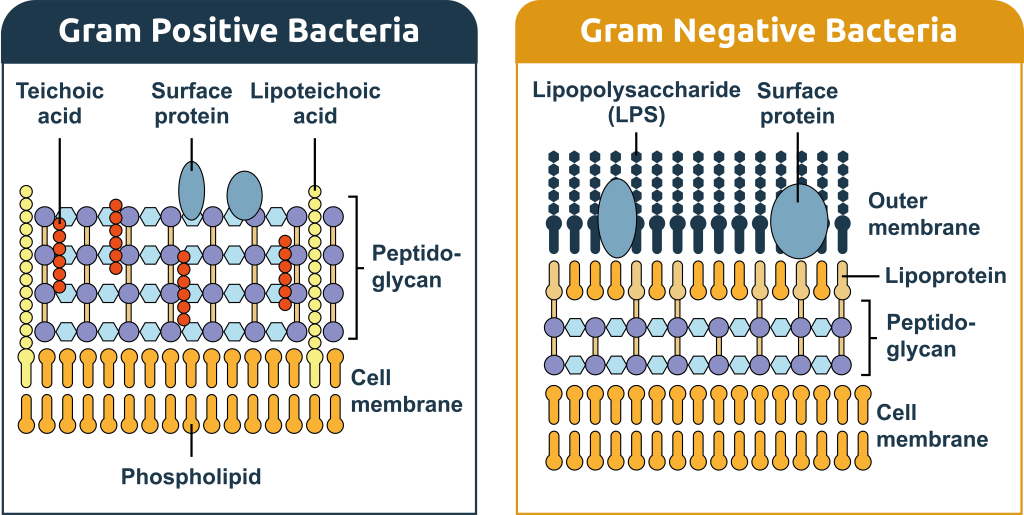
Present only in the outer membrane of gram-negative bacteria, lipopolysaccharides consist of three structural elements (Figure 2).

The physiological activities of LPS are mainly mediated by the lipid A component. Lipid A is a powerful biological pyrogenic that can stimulate the mammalian immune system. Since they are part of the cell wall, LPS probably only exerts their toxic effects when the bacteria lyse (the cell wall splits during autolysis/bacterial growth), with the use of certain types of antibiotics, or when the body’s natural immune response breaks down the cell.
When endotoxins are released from the membrane, their mechanism of action is complex. In humans and mammals, once present in the blood serum, LPS binds to a lipid binding protein (LBP) which transfers it to CD14 receptors on the immune cell membrane. It is then transferred to a non-anchored protein (MD2) which associates with Toll-like receptor-4 (TLR4). A toll-like receptor is a protein, normally found on immune cells, with a single-pass membrane and assists in identifying pathogenic components in the body. CD14 and TLR4 are present in several cells of the innate immune system, including macrophages, monocytes, and dendritic cells.
From here, the signaling cascade is triggered for macrophage and endothelial cells to secrete pro-inflammatory cytokines—IL-1, IL-6, IL-8, tumor necrosis factor (TNF), and platelet-activating factor—and nitric oxide (Figure 3). The release of cytokines then stimulates the production of powerful inflammatory mediators (prostaglandins and leukotrienes). Activation of the complement cascade causes histamine to be released (leading to vasodilation) and results in inflammation and the characteristic ‘endotoxic shock’. The net effect is inflammation, intravascular coagulation, hemorrhaging, and shock.

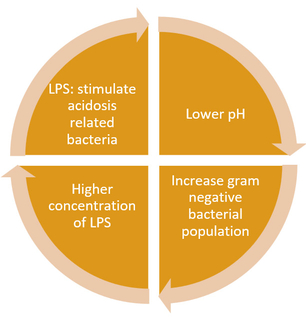
Though all gram-negative bacteria contain endotoxins, the impact or severity of the endotoxemia they cause is affected by several factors, including the type of bacteria, concentration, duration of exposure, the animal’s age, the nutritional/health status of the animal, direct or indirect interaction, as well as if there is a combination of toxins present. The type of exposure is also important, as endotoxins (when excreted) can attach to dust particles and are then inhaled. This latter type of exposure is a risk for both animals and workers.
It is very difficult to assess a “proper level” of endotoxin content, both in the GIT and the blood. The easiest way to sample the GIT content is through feces. Testing feces for endotoxin content is possible, but the tests are expensive and there are no reference values in the scientific literature to assess the health status of the animal based on the endotoxin content of the feces. Nevertheless, we are currently considering trials to determine the effectiveness of endotoxin feces content as a biomarker of the overall endotoxin challenge.
Since only a small number of endotoxins are transferred from the GIT into blood—and once in the blood the endotoxins are quickly metabolized—blood endotoxin levels are extremely variable and depend on the animal and the time of sampling, making it a very unreliable biomarker. Since LBP is produced by the animal as a direct response to an endotoxin challenge, some researchers have suggested using LBP (LPS binding protein) instead. Additionally, the levels of LBP are more stable than those of LPS. However, there is still high variation in the animal and the tests are expensive.
In general, strategies to control endotoxin contamination in animals include all of those aimed at reducing bacterial contamination. These strategies include biosecurity, the use of prebiotics and probiotics, improved nutrient digestibility, etc. Other strategies are vaccinations, immunity modulators, and toxin binders that can target endotoxin contamination.
Some preliminary studies have already tested the possibility of using toxin binders against endotoxins in swine and cattle. Any product considered for endotoxin binding capabilities should be proven with in vitro and in vivo findings. This preliminary data suggests that select toxin binders are capable of binding endotoxins in the gastrointestinal tract, and thus prevent endotoxins from being absorbed. Further studies are currently underway to confirm.
In summary, endotoxins are always present in the GIT of the animal and are often in the upper respiratory tract. Under stress conditions, the microbiota balance of the GIT can be altered towards an increase of gram-negative bacteria, which increases endotoxin content. As a pyrogenic, LPS triggers inflammation pathways that impact animal health and production. Unfortunately, it is difficult to measure endotoxin levels and there are no reference values in the industry.
At the farm level, what is possible to measure is the consequences of an endotoxin challenge on animal performance. As described before, the ultimate effect of an endotoxin presence in the GIT is an inflammatory response. This inflammation has several detrimental effects, like altering the epithelial wall to result in lower nutrient absorption. Additionally, the energy demands from the inflammation process itself consume valuable energy intended for animal health and growth. So, the control of endotoxins—especially during stress situations—could result in improved production parameters.
Different means of controlling endotoxins have been tested but most of them are too expensive to be considered in animal production. A cost-effective method would be the use of toxin binders to capture endotoxins in the gastrointestinal tract and prevent them from entering the bloodstream.
Would you like to be kept informed of our latest developments? Register here and stay up to date.
"*" indicates required fields
Our toxin experts are here to answer questions, give expert advice, or guide you to an optimal management solution.
Reach out now!
Do you have a question? Our Agrimprove experts are willing to help.
"*" indicates required fields
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
