The importance of endotoxins in poultry production is a subject of much interest in some regions, as a result of marketing in the recent years of several products having positive effects in birds, while other works showed adverse effects in other species.
.
Something we quickly detected when reviewing some of these reports indicating effectiveness in birds, is the absence of measurements indicating that endotoxins were actually present in the animals evaluated. Therefore, it is worth asking,
“How can we associate improvements in production parameters when using these additives if they do not detect the presence, absence or decrease in the level of endotoxins in treated animals??”
We fully understand that a major limitation in detecting whether these toxins are present in animals is being able to use reliable laboratory tests to measure their concentration in blood, urine or feces.
They are structural components of Gram Negative type bacteria, regardless of pathogenic or non-pathogenic, consisting of protein material and fats that form the outer membrane of the bacterial cell wall.
Gram Negative bacteria have a cell envelope that contains three layers or membranes:
Since Gram-positive bacteria do not have an outer membrane, they do not contain endotoxins.
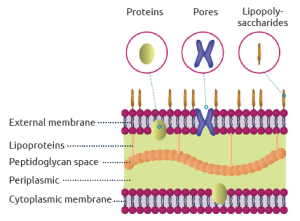
Figure 1: Structural diagram of Gram-negative bacteria
Endotoxins is used to describe a complex containing lipopolysaccharides (LPS) associated with the outer membrane of bacteria such as Eschericia coli, Salmonella, Pseudomonas and Pasteurella, among others.
The most important bacteria that contains endotoxins, belong to the group Enterobacteriaceae and in many cases are part of the normal intestinal microbial flora of birds and mammals (including humans).
LPS are composed of three elements: Lipid A, nucleus and O antigen.
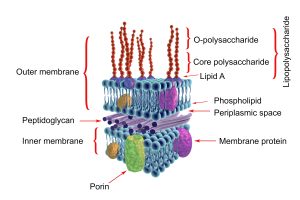
Figure 2. Important structures in a Gram-negative bacterium.
Two mechanisms have been detected:
1. Production during the initial phase of bacterial growth, both in the laboratory (in vitro) and in animals (in vivo).
2. Product of the destruction (lysis) of bacteria by the effect of the immune system or antimicrobial agents
When animals are exposed to an excessive level of stress, the concentration of free endotoxins in the body increases. Once the balance established in the intestinal microbial flora is lost (dysbacteriosis or dysbiosis), the final result is the development of Salmonellosis and/or Colibacillosis. In other words, the absence of
some beneficial bacteria, for example, Lactobacillus, will allow pathogenic bacteria to grow in the intestines.
It is important to differentiate endotoxins from exotoxins. The latter are produced by both Gram negative and
positive bacteria, with a highly specific effect on the host.
Unlike endotoxins, exotoxins are secreted by bacteria and small amounts are very lethal, as in the case of botulism and secretions can be converted to toxoids, as in the case of the tetanus vaccine, produced by Clostridium tetani.
For decades, deleterious effects have been observed when injecting bacterins prepared with Gram-negative pathogens. Scientific publications have shown that by injecting small concentrations of endotoxins from Pasteurella multocida into broilers, the clinical signs of acute cholera can be reproduced, without the need to challenge with the whole bacteria.
The best example of this type of damage at a commercial level, is caused by the injection of bacterins against Fowl Cholera, characterized by causing depressed birds after its application in pullets during the rearing period.
On the other hand, the reactions at the application site are very important due to seizures at the slaughterhouse level, especially when using oil emulsions. A recommendation by biological companies that illustrates the importance of endotoxins when vaccinating, is to keep vaccines at an average temperature of approximately 37 ºC because overheating causes the release of endotoxins. The release of endotoxins present in the vaccine causes severe reactions and mortality and can cause a hemorrhagic syndrome.
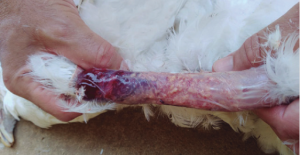
Photo 1. Local reaction at the application site of a bacterin injected into the neck of a 12-week-old commercial pullet, probably caused by endotoxins (LPS). The photo was taken at 18 weeks and shows yellowish caseous material of bacterial origin due to needle or syringe contamination.
The most important thing is to determine if they are actually causing any harm to birds, before investing in prevention. If a real effect is determined, the following strategies can be used.
In conclusion, despite the fact that the adverse effects caused by endotoxins in pigs and dairy cows seem to be more defined, in the case of poultry their negative impact is not categorically demonstrated.

Would you like to be kept informed of our latest developments? Register here and stay up to date.
"*" indicates required fields
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
